- Introduction
- Data Coverage
- Further Details
- Request Access
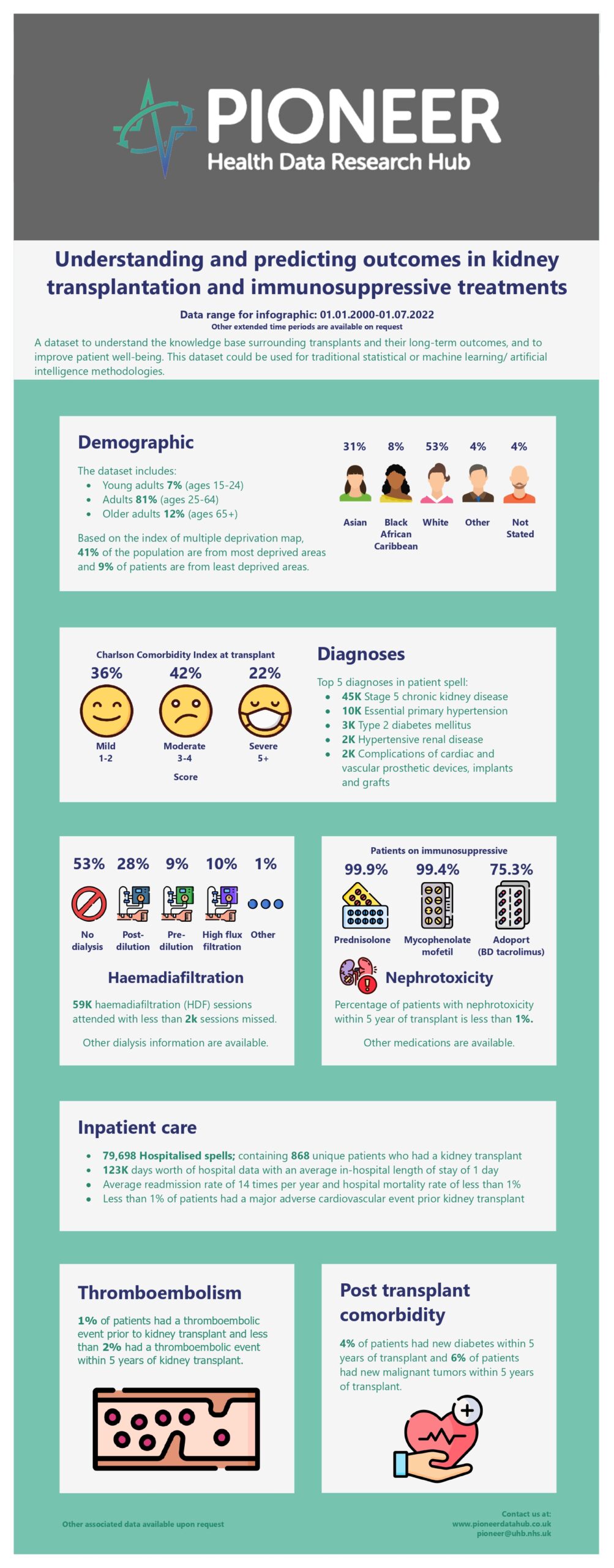
A highly granular dataset of 868 patients on immunosuppressive treatments who underwent a kidney transplant. The data includes demography, serial physiology, assessments, diagnostic codes (ICD-10 & SNOMED-CT), initial presentation, presenting symptoms, dialysis, post-transplant chronic kidney disease, procedures (OPCS4 & SNOMED-CT), imaging, prescriptions, outpatient appointments and outcomes. Dataset also includes acute presentations, with opportunities to learn the drivers of emergency admissions in this group. The current dataset includes admissions from 1st January 2000 to 1st July 2024 to observe and improve the long-term outcomes of patients but can be expanded to assess other timelines of interest. End-Stage Renal Disease (ESRD) is the advanced stage of kidney disease requiring renal replacement therapy. Kidney transplantation offers improved outcomes but requires immunosuppressive treatment to prevent organ rejection. However, these medications pose risks such as infections and metabolic complications, highlighting the complexity of managing ESRD post-transplantation. 
Geography: The West Midlands (WM) has a population of 6 million & includes a diverse ethnic & socio-economic mix. UHB is one of the largest NHS Trusts in England, providing direct acute services & specialist care across four hospital sites, with 2.2 million patient episodes per year, 2750 beds & > 120 ITU bed capacity. UHB offers one of the largest renal transplant services nationally. UHB runs a fully electronic healthcare record (EHR) (PICS; Birmingham Systems), a shared primary & secondary care record (Your Care Connected) & a patient portal “My Health”. Data set availability: Data access is available via the PIONEER Hub for projects which will benefit the public or patients. This can be by developing a new understanding of disease, by providing insights into how to improve care, or by developing new models, tools, treatments, or care processes. Data access can be provided to NHS, academic, commercial, policy and third sector organisations. Applications from SMEs are welcome. There is a single data access process, with public oversight provided by our public review committee, the Data Trust Committee. Contact pioneer@uhb.nhs.uk or visit www.pioneerdatahub.co.uk for more details. Available supplementary data: Matched controls; ambulance and community data. Unstructured data (images). We can provide the dataset in OMOP and other common data models and can build synthetic data to meet bespoke requirements. Available supplementary support: Analytics, model build, validation & refinement; A.I. support. Data partner support for ETL (extract, transform & load) processes. Bespoke and “off the shelf” Trusted Research Environment (TRE) build and run. Consultancy with clinical, patient & end-user and purchaser access/ support. Support for regulatory requirements. Cohort discovery. Data-driven trials and “fast screen” services to assess population size.



