- Introduction
- Data Coverage
- Further Details
- Request Access
The incidence of blood clots in the lungs (pulmonary embolus or PE) or limbs (deep vein thrombosis or DVT) is estimated to be approximately 50–150 per 100,000 people and in the UK, around 60,000 cases of PE and 200,000 cases of DVT are reported each year. Emergency admissions for PE increased by 30% between 2018 and 2022. However, for every PE or DVT suspected, only 25% of patients are confirmed to have the diagnosis. Despite significant progress, diagnosing PE and DVT remains a challenge. This large synthetic data with up to 14.5k patients of both suspected and diagnosed thromboembolic events provides key parameters to support critical research into the condition. 
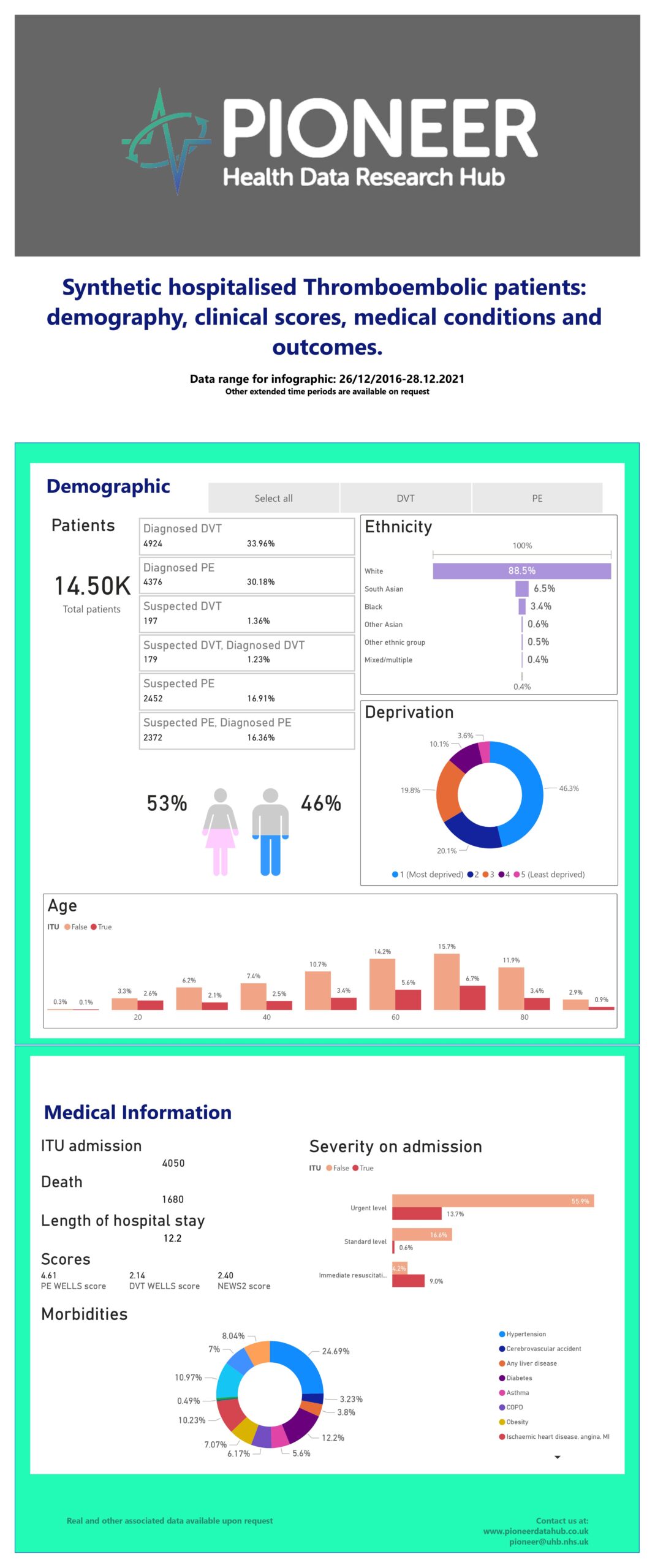
Geography The West Midlands (WM) has a population of 5.9 million & includes a diverse ethnic & socio-economic mix (42% non-white within Birmingham). EHR UHB is one of the largest NHS Trusts in England, providing direct acute services & specialist care across four hospital sites, with 2.2 million patient episodes per year, 2750 beds & an expanded 250 ITU bed capacity during COVID. UHB runs a fully electronic healthcare record (EHR) (PICS; Birmingham Systems), a shared primary & secondary care record (Your Care Connected) & a patient portal, “My Health”. Scope Enabling data-driven research and machine learning models towards improving the diagnosis of Thromboembolic events (PE/DVT). Real-world dataset linked. The dataset includes large patient demographics, clinical scores, and medical conditions for PE/DVT patients, alongside outcomes taken from ICD-10 & SNOMED-CT codes. Available Supplementary Data Real-world PE/DVT cohort. Available Supplementary Support Analytics, model build, validation & refinement; A.I., data partner support for ETL (extract, transform & load) process, clinical expertise, patient & end-user access, purchaser access, regulatory requirements, data-driven trials, “fast screen” services.



